This fact sheet provides information on sharps devices with specific engineering controls designed to reduce the risk of sharps injury and minimize occupational exposure to bloodborne pathogens and biohazardous materials in research laboratories.
Why do we need sharps protection?
At the University of Utah there are numerous sharps injuries each year, resulting in potential exposure of personnel to Hepatitis B virus, Hepatitis C virus or Human Immunodeficiency Virus (HIV) and other bloodborne pathogens.. The CDC estimates that 62–88% of sharps injuries can be prevented by the use of safer sharps. The University of Utah/OSHA Bloodborne Pathogens Standard requires the use of safer sharps devices whenever possible.
Safer sharps devices have built-in engineering controls to prevent sharps injuries. Devices that reduce the time that a sharp edge or point is exposed (e.g., retractable or single-handed self-sheathing needles or blade devices), substitute plastic for glass or increase the distance between the sharp edge/point to the user are potential safer sharps devices.
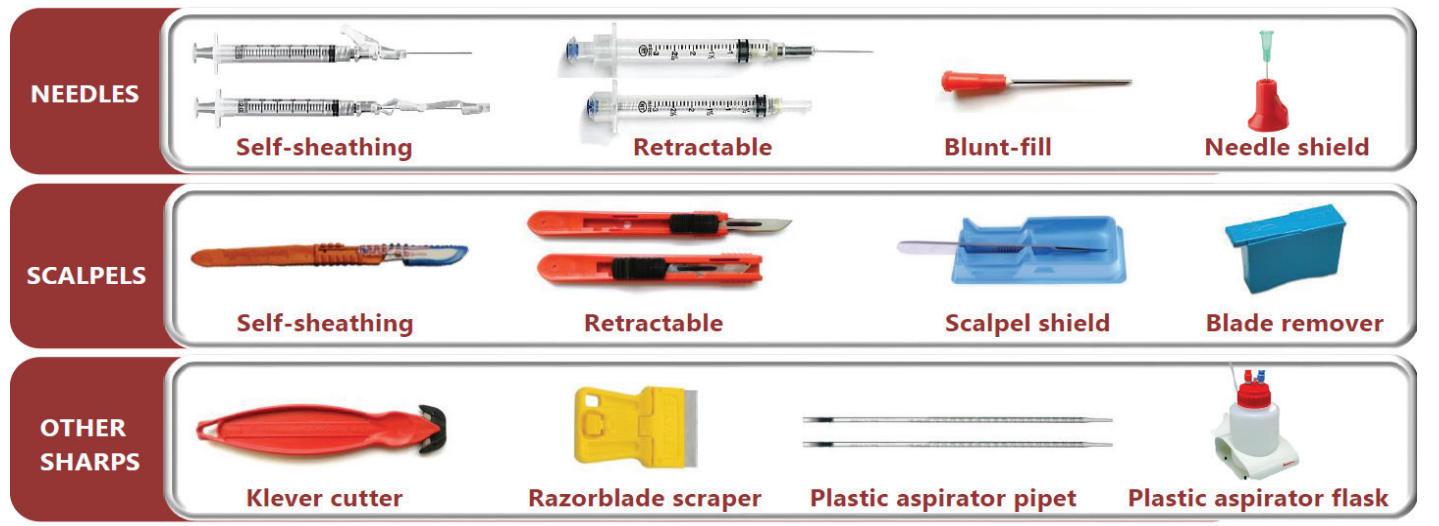
Safer needle devices have single-handed activation mechanisms for injections. Blunt-fill needles penetrate a rubber septum but require 10× force to pierce skin. Safer blade devices use a manual mechanism to retract or shield the blade. Klever cutters have an integrated shielded blade for opening boxes. Replace glass items with plastic alternatives whenever feasible. Use cut- or stick-resistant PPE as a last line of defense.
Injection
- Self-sheathing needle
- Retractable needle
Dissection
- Retractable Scalpel
- Blunt-Tipped forceps

Cutting Gels
- Razorblade Scraper
- Spatula

Media Aspiration
- Plastic aspirator pipet
- Plastic aspirator flask
Visit OEHS Examples of Sharps Protection Fact Sheet for a list of vendor and product information.
Adapted from UCLA EH&S Guidance
